Jay Yang
Singer Instruments, Roadwater, UK, TA23 0RE
Summary
The yeast 2-hybrid (Y2H) assay is a well-established technique to detect protein-protein interactions. This is an extremely powerful tool for researchers and is often used alongside one or two other methods to examine the multitude of interactions that take place in cells. The assay is straightforward to perform and generates high-quality results in a short amount of time.
Principles
- Y2H assay relies on the expression of a reporter gene (such as lacZ or GFP), which is activated by the binding of a particular transcription factor.
- The transcription factor is comprised of a DNA-binding domain (BD) and an activation domain (AD).
- The query protein of interest fused with the BD is known as the Bait, and the protein library fused with the AD is referred to as the Prey.
- To activate the reporter gene expression, a transcriptional unit must be present at the gene locus, which is only possible if Bait and Prey interact.
Theory
Expression of a reporter gene requires the binding of a transcription factor, which normally consists of two functionally and structurally independent domains: DNA-binding (DB) and activation (AD) domains. The DB domain binds to the particular DNA sequence upstream of the reporter gene, while the AD domain activates reporter gene expression (Figure 1A).
Since AD and DB domains are functionally and structurally independent, they can be fused to two separate proteins. The protein that is fused to the DB domain is called the Bait, while the one fused to the AD domain is referred to as the Prey.
In the absence of Bait-Prey interaction, the AD domain is unable to localize to the reporter gene to drive gene expression (Figure 1B). However, when Bait and Prey interact, the DB domain binds to the DNA localizing the AD domain upstream of the reporter gene, leading to the expression of the reporter gene [1] (Figure 1C).
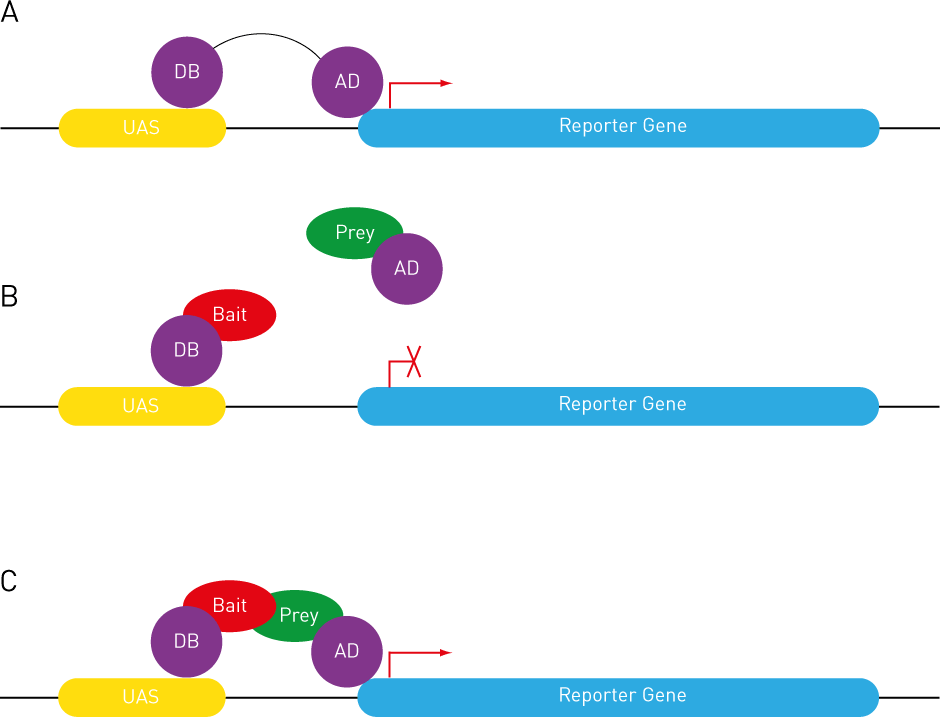
Figure 1: Y2H Principles. See text for details.
Yeast 2-Hybrid Variations
Several variations of the yeast 2-hybrid assay have been developed in order to study different protein interactions.
The ROTOR+ is the fastest and most powerful colony manipulation robot in the world. It is essential for any large-scale Y2H studies.
Yeast 1-Hybrid
The Yeast 1-hybrid assay examines protein-DNA interactions [2].
A query protein is directly fused with the AD domain and expressed in yeast strains harbouring various target DNA sequences upstream of the reporter gene. Thus, if the query binds to a particular target sequence, the associated AD domain will activate reporter gene expression (Figure 2A).
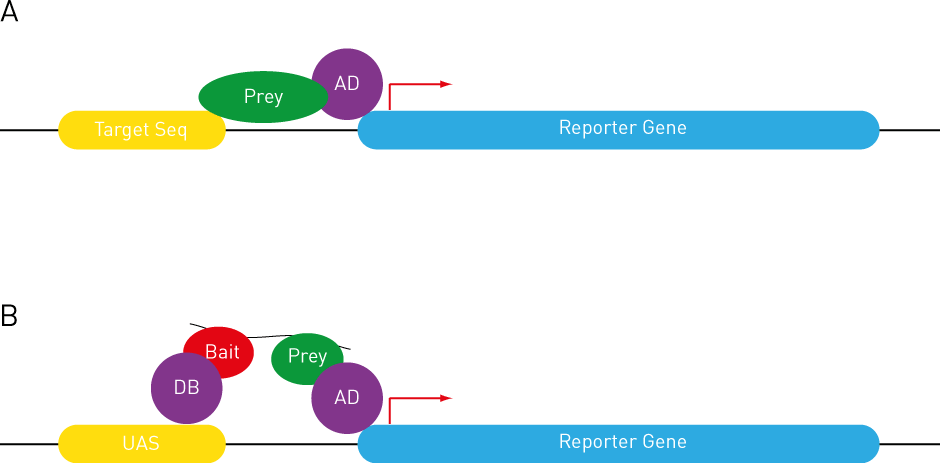
Figure 2: Variations of the Y2H Assay
(A) Yeast 1-hybrid assay. (B) Yeast 3-hybrid assay. See text for details.
Yeast 3-Hybrid
Yeast 3-hybrid assays study protein interactions that are mediated by a third component, such as an RNA molecule or another protein [3].
In this instance, Bait and Prey do not directly interact with each other. Instead, they bind to, for example, an RNA molecule, albeit with different sequence specificity. Therefore, only in the presence of the particular RNA molecule would Bait and Prey be able to interact and drive reporter gene expression (Figure 2B).
Split Ubiquitin Yeast 2-Hybrid
The split ubiquitin assay identifies non-soluble membrane protein interactions [4].
Instead of fusing to AD and DB domains, query proteins are fused with one of the following two ubiquitin moieties: C-terminal ubiquitin moiety (Cub) and N-terminal ubiquitin moiety (Nub). The Cub is also fused with a transcription factor that can be cleaved by the specific protease (Figure 3A).
The interaction of the query proteins allows the reconstitution of the ubiquitin, which in turn, is recognized by the protease (Figure 3B). Cleavage of the protease will release the transcription factors to the nuclear, thereby activating the reporter gene expression (Figure 3C).
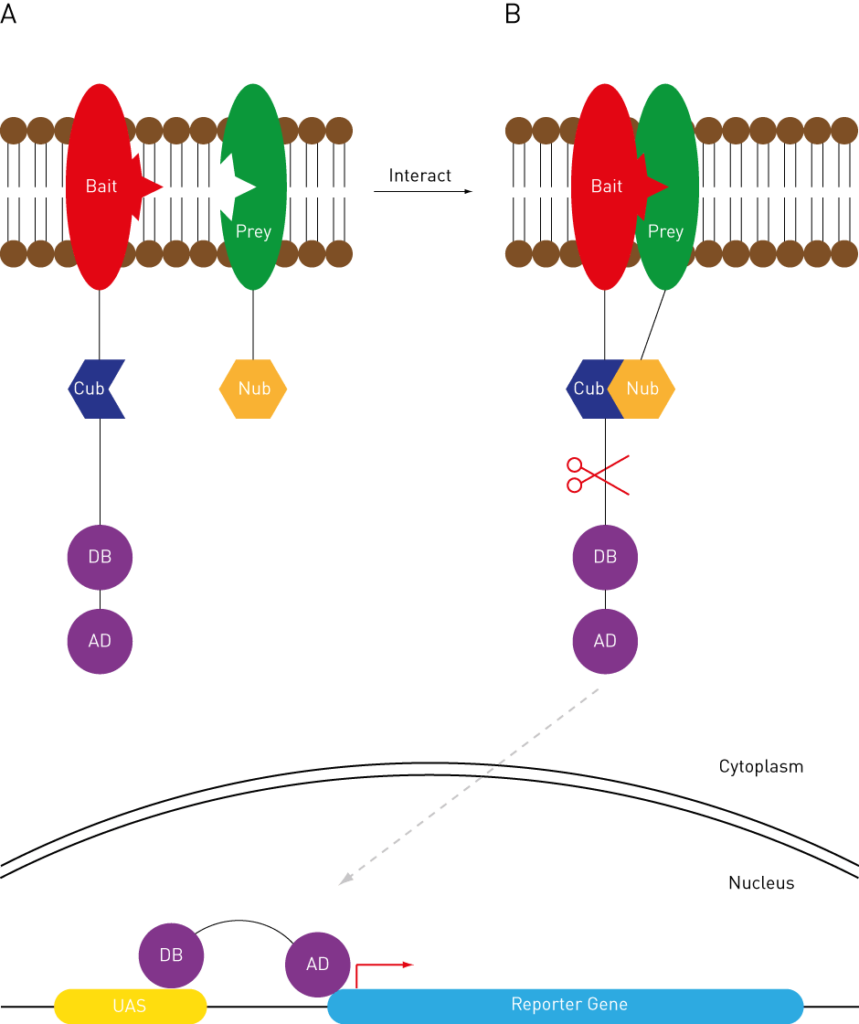
Figure 3: Split Ubiquitin Y2H. See text for details.
Advantages and Limitations
Yeast 2-hybrid is a powerful technique to identify protein interactions because of its straightforward methodology and fast turnaround time. Therefore, the throughput of the technique can be scaled up significantly to screen the entire proteome [5]. In addition, the technique has been adapted in other model organisms to study organism-specific interactions [6].
It is important to recognize the limitations of yeast 2-hybrid assays:
- The assay can produce a high level of false positive and negative interactions. This is an important reason to validate any interactions using other techniques such as co-immunoprecipitation.
- Interaction must occur in the nucleus of the cell in order for the reporter gene to be activated. Proteins that are localized to other cellular compartments may not produce a positive interaction, even if they interact directly. This can be overcome by using a split ubiquitin 2-hybrid system or by performing the assay in bacteria, which do not possess a nucleus.
- Overexpression of recombinant fusion protein, which happens in most yeast 2-hybrid experiments, could produce spurious interaction data. In addition, the fusion of AD/DB domains to query proteins may affect query protein function in vivo.
- Query proteins may not be correctly expressed, folded, or modified when expressed in yeast. Therefore, it is important to confirm that query proteins are functional before deriving interaction data from the assay.
Working with Libraries
The availability of the Y2H Bait library collection has drastically elevated the throughput of the assay [7]. A single Bait strain can be systematically crossed to an ordered array of yeast strains expressing individual Prey proteins, allowing proteome-wide screening and identification of novel protein interactions.
High-throughput screening using such collections can be done quickly and easily using automated robotics, such as the Singer ROTOR+, which can rapidly pin high-density arrays of yeast and facilitate strain mating and selection. In addition, the ROTOR+ screening robot provides an automated platform to produce multiple experimental replicates simultaneously.
Conclusion
Gene regulatory networks (GRNs) comprising interactions between transcription factors (TFs) and regulatory loci control development and physiology. Numerous disease-associated mutations have been identified, the vast majority residing in non-coding regions of the genome. As current GRN mapping methods test one TF at a time and require the use of cells harbouring the mutation(s) of interest, they are not suitable for identifying TFs that bind to wild-type and mutant loci. Here, we use gene-centred yeast one-hybrid (eY1H) assays to interrogate the binding of 1,086 human TFs to 246 enhancers, as well as to 109 non-coding disease mutations. We detect both loss and gain of TF interactions with mutant loci that are concordant with target gene expression changes. This work establishes eY1H assays as a powerful addition to the toolkit for mapping human GRNs and for the high-throughput characterization of genomic variants that are rapidly being identified by genome-wide association studies.
Y2H Applications
Human Gene-Centered Transcription Factor Networks for Enhancers and Disease Variants
Abstract (Fuxman Bass JI, Sahni N, Shrestha S, Garcia-Gonzalez A, Mori A, Bhat N, Yi S, Hill DE, Vidal M, Walhout AJ)
References
Kraemer B, Zhang B, SenGupta D, Fields S, Wickens M (2000) Using the yeast three-hybrid system to detect and analyze RNA-protein interactions. Methods in enzymology 328: 297-321.
Walhout AJ, Boulton SJ, Vidal M (2000) Yeast two-hybrid systems and protein interaction mapping projects for yeast and worm. Yeast 17: 88-94.

