In this case study, Prof. Kyle Lauersen explains why ROTOR+ and PIXL are together essential to meet his lab’s high-throughput screening needs.
Kyle leads the Sustainable & Synthetic Biology (SSB) group, in the Biological and Environmental Science and Engineering (BESE) Division, at King Abdullah University of Science and Technology (KAUST). His research group aims to develop resource-efficient bio-processes involving genetically engineered algae.
Engineering these organisms allows us to tailor algal metabolism to produce non-native compounds, by bringing pathways from other organisms, like plants and fungi, into the alga. Once there, the pathways reroute native metabolism to produce products of interest, all from carbon dioxide.

Despite being in the lab since the 1940s, this green alga has complicated genetics. Since 2015, we’ve known the trick for working with the organism’s native gene regulation machinery to reliably express transgenes from its nuclear genome. However, engineering algae is a numbers game, because DNA randomly integrates into the genome, causing a range of expression across a population of transformed cells. So we need to screen hundreds of transformants to find the one we want.
Our previous workflows involved the manual picking of transformed colonies with toothpicks. As one can imagine, this is a very tedious process, and when there are 20 different plasmids to try, picking hundreds of colonies manually for each one can take a group of students several finger breaking days.
The PIXL increases our throughput (and sanity) by over 8-fold. In addition to that, the standardisation of colony arrays made by the robot are far superior in quality and reproducibility than anything we could do by hand. We are able to reduce media use, as we can get many many more colonies per plate area than by manual picking. The maximum we trust ourselves to do by hand is 144 colonies per 13×13 cm plate, while the PIXL easily puts 384 colonies on its small rectangular plates.
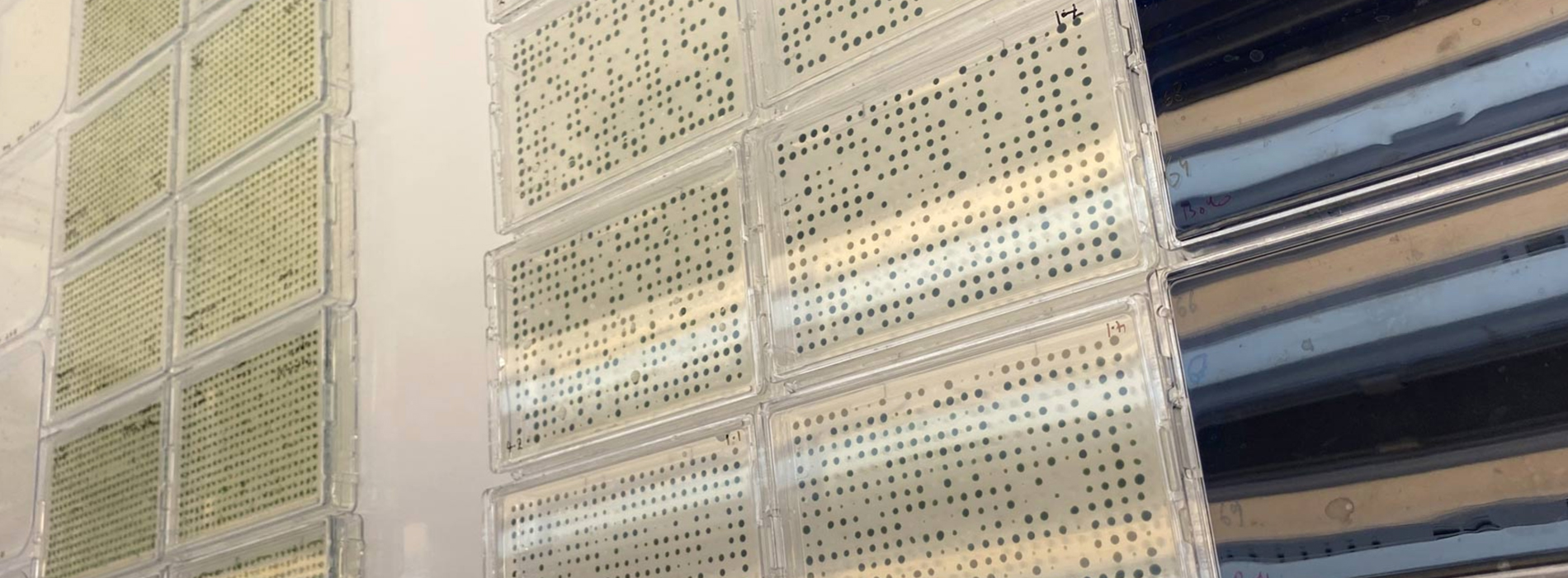
Algal colonies are not simple to store by freezing, and we maintain our collections on agar plates which get refreshed bi-weekly or monthly. The ROTOR+ then allows rapid, clean, and reliable colony maintenance and media switching. Again, our normal workflow is to use the back of PCR plates as a stamp to transfer colonies from one agar plate to another. This, obviously has its limitations, both in human error and the fact that only 96 colonies can be transferred at a time. The ROTOR+ removes all artisanal human error from the stamping process while enabling much higher densities of colony transfer in any situation. It also allows rapid inoculation of 96/384 well liquid plates, using the long pins. This standardisation process also allows us to improve the analysis of colony features statistically across a population already at the agar plate level.
“I can’t speak highly enough about these two instruments. We have had them up and running for 6 months in our lab, and together, they have been a game-changer in terms of throughput and reproducibility.”

Prof. Kyle Lauersen