For over 80 years Singer Instruments have been dedicated
to making instrumentation to accelerate science.
We do this because we really believe in the importance of the scientific research that you do.

Need to boost your screening throughput?


Want to automate your colony picking workflows?

Looking for validation? Find white papers, user testimonials, application notes, videos and more.
Seeking technical or application support from a qualified engineer or biologist?
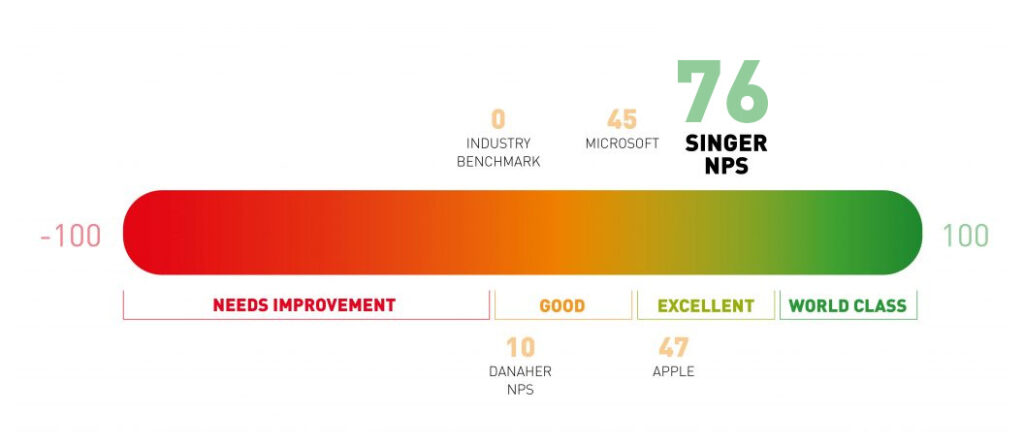
We strive for your happiness in everything that we do, from our first interaction to 20 years after your installation

>1,000 Installations Worldwide
Singer Instruments support labs across 60 countries: in North America, Europe, Asia, Oceania, South America, and Africa.

10,000 Citations and Counting
The mission is ‘To develop laboratory automation to accelerate research for scientists who want to make the world a better place.’
We think >10,000 citations is a good start, but we’re far from finished.

Trusted by FTSE 100 Companies
Our products are helping to accelerate science in labs at: NASA, Apple, Carlsberg, the FBI, Facebook, Pfizer, and the top 50 Universities in the World.*