Report on mixing efficacy of ROTOR+
Biological testing was performed on ROTOR+ to demonstrate the effectiveness of the system’s ability to consistently and efficiently transfer biological material. The results confirm that utilising the ROTOR+s 3D mixing capability ensures complete and consistent transfer of Saccharomyces cerevisiae colonies, achieving a 100% transfer rate. In contrast, control experiments performed without the mixing function showed poor and inconsistent results.
Introduction
For the past 20 years, ROTOR(+) series have been the reliable workhorse of countless laboratories worldwide. Continuous improvements saw it expand from agar-agar Synthetic Genetic Array (SGA) workflow automation to include versatile and complex high-throughput screens for genetic interaction mapping, strain engineering/optimisation and adaptive laboratory evolution experiments.
With its ability to screen almost a million colonies per hour, it’s highly adapted to a range of molecular biology workflows for both model and non-model microbial organisms. ROTOR+ has a reputation for its precision, reliability and unparalleled ability to consistently transfer biological material while high-density arraying. This is owed in large part to its range of advanced user-defined parameters for treatment of both source and destination plates, including mixing.
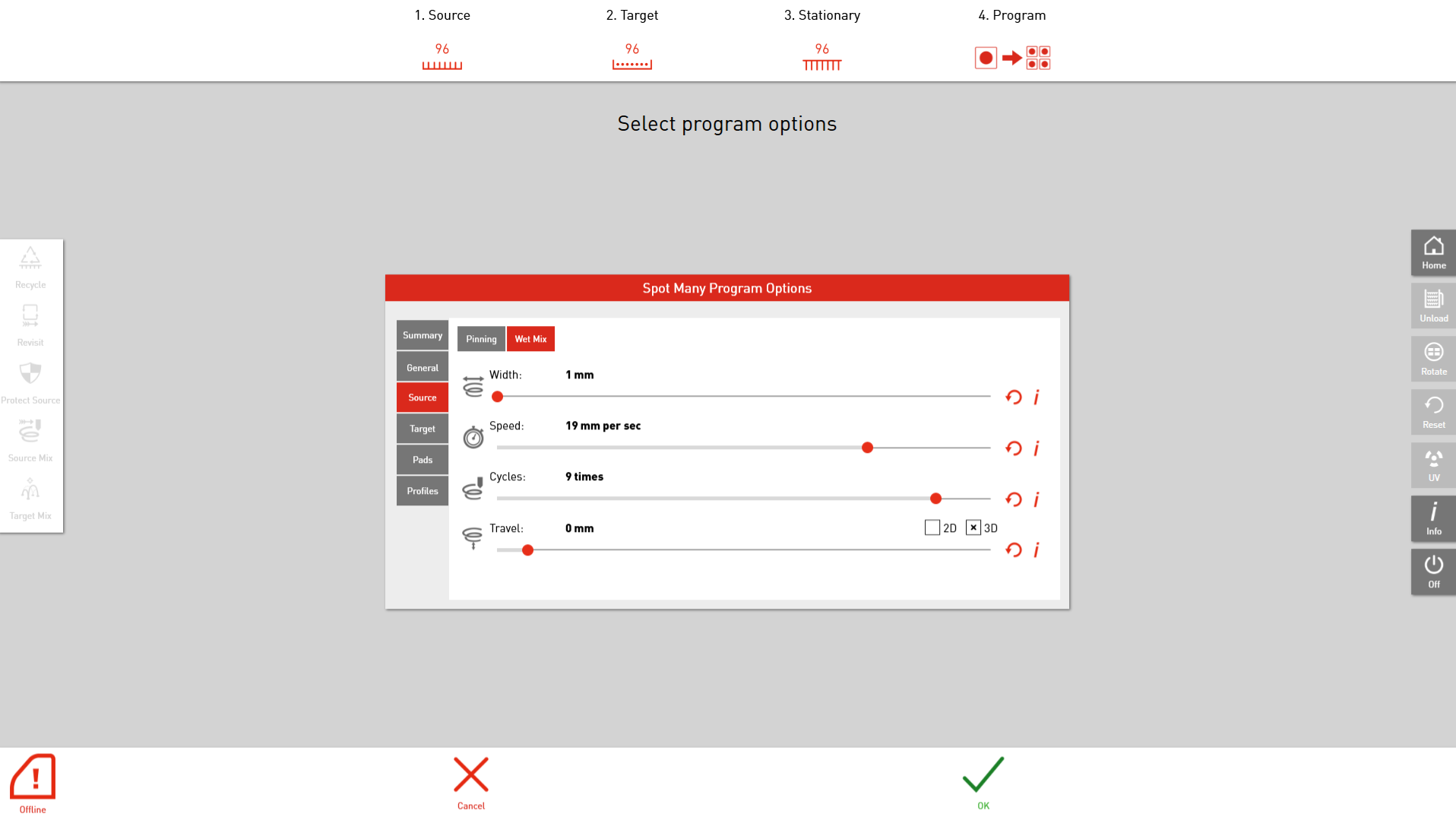
ROTOR+’s easy-to-use user interface to control the mixing parameters. Control the width, speed, number of cycles and travel depth with ease.
This report illustrates the essential role of ROTOR+’s 3D mixing capability in achieving complete and consistent transfer efficiency of biological material for maximum experimental robustness and reproducibility.
Method
The efficacy of the ROTOR’s mixing capability was assessed using a series of transfers from multi-well plates.
All plates were incubated overnight at 30 °C , and results were assessed via qualitative visualisation. Multi-well plates used in this experiment were Nunc™ MicroWell™ 96-Well, Nunclon Delta-Treated, Flat-Bottom Microplates.
- A donor plate containing a 96-array of Saccharomyces cerevisiae (BY4741) colonies was prepared on a 2% YPD agar plate.
- The inside of ROTOR+ was wiped with 2% Distel and the ROTOR+ UV cycle was run for 60 minutes prior to use to aid sterilisation.
- ROTOR+ was used to stamp (agar-to-liquid) the donor plate into multi-well plates (MWPs) containing sterile deionised water, with the target plate 3D-mix enabled. This was performed in triplicate.
- The freshly inoculated liquid MWPs were then spotted (liquid-to-agar) onto blank agar plates, this time with the source plate 3D mixing enabled. The spotting was performed in technical duplicates for each biological replicate.
- Control experiments were included where no mixing was applied during the transfer steps to directly compare efficacy.
- All plates were incubated overnight at 30 °C , and results were assessed via qualitative visualisation.
Results
Colonies of S. cerevisiae BY4741 were successfully transferred 100% of the time when mixing settings were applied. In comparison, without mixing enabled, the amount of biological matter transferred to the destination plates was repeatedly inconsistent (Figure 1).
The 3D mix parameters were set as follows:
- Speed 19 mm/sec
- Backoff 0.5 mm
- Repeat pin 3 times
- Wet mix diameter 1 mm
- Wet mix speed 24 mm/sec
- Wet mix cycles 9 times
- Travel 1mm
- 3D mixing ON
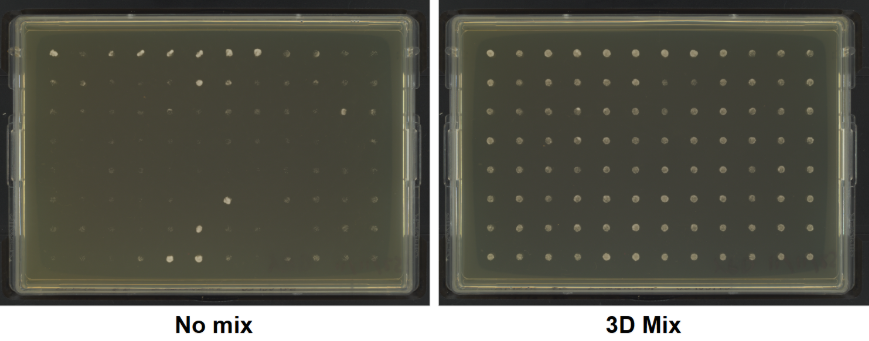
Figure 1. The 3D mixing functionality in ROTOR models enhances the transfer efficiency of biological material. Colonies of S. cerevisiae BY4741 were successfully transferred 100% of the time when 3D mixing was applied (right). Conversely, uneven depositing of biological material was observed when no mixing was applied (left).
Looking to plan a high throughput workflow using ROTOR+?
The ROTOR+ supports scientists to find that 1 colony in a million, in one day.
That’s why we have 10,000 citations and counting

Marina Serdar | Product Manager (PhD, BSc)
Driven by a desire to directly impact the work of her fellow microbiology researchers, Marina transitioned from the academic lab to the dynamic world of industry. Her journey at Singer Instruments began as Product Owner, where she guided the interdisciplinary team responsible for bringing the high-resolution, high-throughput colony imager, ColonyCam, from initial concept to production. Now, she’s expanded her scope, taking on the mantle of Value Stream Owner for High-Density Array and Replication while also overseeing the broader product portfolio. Marina’s passion lies in prioritising both scientific rigor and user experience, ensuring that Singer Instruments’ products empower researchers to push the boundaries of discovery.