Tom Drake, Singer Instruments, Roadwater, UK. 01/03/2019
Summary
This report covers the measurement and evaluation of the pinning pressure achieved by the PIXL Precision Colony Picker, produced by Singer Instruments.
The pinning pressure was measured using a bespoke test procedure, which replicated the standard pinning functionality of the PIXL. Over the span of 80 separate pinning events, the PIXL achieved an average pressure of 6.97 ± 0.74 g/mm2, with a maximum recorded pressure of 8.531 g/mm2.
Introduction
When Singer Instruments was asked by their customers to develop a colony picker, one of the main requirements was to outperform the achievable precision and contamination rates of pneumatic firing pins. There are serious concerns that accelerated pin firing damages and/or misses important samples and can contribute to contamination. When designing PIXL, one of the primary considerations was the maximum speed of approach to ensure no chance of microbial material becoming airborne and causing contamination; or cell splashing. Meticulous analysis identified the maximum speed of approach to be 19mm/s to ensure no cell splashing, and therefore this is the default approach speed for PIXL.
PIXL uses a novel polymer (PickupLine™) to pick microbial colonies and deposit them on a variety of target plates. The pressure applied to agar plates (and colonies) is vital to ensure agar is not pierced, this guarantees that picking is repeatable and that colonies are not damaged. PIXL was designed to have a gentle picking force and therefore pressure, to never pierce agar. We have minimised the chance of contamination through our regulated speed of approach and pressure. A high-speed of approach could induce contamination if cells are forcibly hit by a firing pin causing cell splashing. Regulating contact pressure on every pick is vital to ensure that agar depth can vary across plates and that contamination is minimised whilst protecting microbial samples.
Method
To measure the pinning pressure delivered by PIXL, a laboratory scale was used. This was positioned at the source bay location replicating the location of a source plate. The height was representative of an agar-filled PlusPlate. For the testing, a routine was produced that would hold the applied weight to allow a reading to be taken from the scale.
Every applied parameter in this routine was set to default to represent a typical picking run. The applied weights were recorded for eighty consecutive pinning events to give a representative sample.
The recorded weights were then converted to pressure, accounting for the diameter of PickupLine as 1mm.
Results
The data from eighty consecutive pinning events is presented in Figure 1, with the raw data and calculated pressure shown in Table 1 (below).
Each data point recorded is detailed below in Table 1. To account for the diameter of the PickupLine, a surface area of 0.785 mm2 was used to calculate surface pressure.
A mean pressure of 6.97 g/mm2 was calculated, with a standard deviation of ±0.74 g/mm2. The maximum pressure observed was 8.79 g/mm2 and the minimum pressure observed was 5.22 g/mm2.
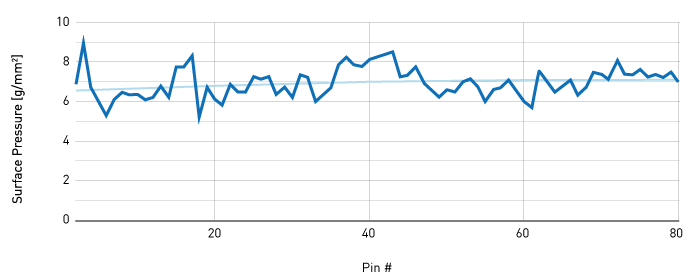
Figure 1 – Calculated pressure values for all pinning events. All eighty calculated pressure values are shown with their corresponding pin number. An average is shown and suggests there is no increase over time.
Table 1
Complete raw weight and calculated pressure for all recorded pinning events. All eighty data points are shown in full below, with corresponding ID, raw weight recorded, and calculated pressure values.
| ID | Weight [g] | Pressure [g/mm2] | ID | Weight [g] | Pressure [g/mm2] | ||
| 1 | 5.4 | 6.875 | 41 | 6.6 | 8.403 | ||
| 2 | 6.9 | 8.785 | 42 | 6.7 | 8.531 | ||
| 3 | 5.3 | 6.748 | 43 | 5.7 | 7.257 | ||
| 4 | 4.8 | 6.112 | 44 | 5.8 | 7.385 | ||
| 5 | 4.2 | 5.348 | 45 | 6.1 | 7.767 | ||
| 6 | 4.8 | 6.112 | 46 | 5.5 | 7.003 | ||
| 7 | 5.1 | 6.494 | 47 | 5.2 | 6.621 | ||
| 8 | 5.0 | 6.366 | 48 | 4.9 | 6.239 | ||
| 9 | 5.0 | 6.366 | 49 | 5.2 | 6.621 | ||
| 10 | 4.8 | 6.112 | 50 | 5.1 | 6.494 | ||
| 11 | 4.9 | 6.239 | 51 | 5.5 | 7.003 | ||
| 12 | 5.3 | 6.748 | 52 | 5.6 | 7.130 | ||
| 13 | 4.9 | 6.239 | 53 | 5.3 | 6.748 | ||
| 14 | 6.1 | 7.767 | 54 | 4.7 | 5.984 | ||
| 15 | 6.1 | 7.767 | 55 | 5.2 | 6.621 | ||
| 16 | 6.5 | 8.276 | 56 | 5.3 | 6.748 | ||
| 17 | 4.1 | 5.220 | 57 | 5.6 | 7.130 | ||
| 18 | 5.3 | 6.748 | 58 | 5.2 | 6.621 | ||
| 19 | 4.8 | 6.112 | 59 | 4.7 | 5.984 | ||
| 20 | 4.6 | 5.857 | 60 | 4.5 | 5.730 | ||
| 21 | 5.4 | 6.875 | 61 | 5.9 | 7.512 | ||
| 22 | 5.1 | 6.494 | 62 | 5.5 | 7.003 | ||
| 23 | 5.1 | 6.494 | 63 | 5.1 | 6.494 | ||
| 24 | 5.7 | 7.257 | 64 | 5.4 | 6.875 | ||
| 25 | 5.6 | 7.130 | 65 | 5.6 | 7.130 | ||
| 26 | 5.7 | 7.257 | 66 | 5.0 | 6.366 | ||
| 27 | 5.0 | 6.366 | 67 | 5.3 | 6.748 | ||
| 28 | 5.3 | 6.748 | 68 | 5.9 | 7.512 | ||
| 29 | 4.9 | 6.239 | 69 | 5.8 | 7.385 | ||
| 30 | 5.8 | 7.385 | 70 | 5.6 | 7.130 | ||
| 31 | 5.7 | 7.257 | 71 | 6.3 | 8.021 | ||
| 32 | 4.7 | 5.984 | 72 | 5.8 | 7.385 | ||
| 33 | 5.0 | 6.366 | 73 | 5.8 | 7.385 | ||
| 34 | 5.3 | 6.748 | 74 | 6.0 | 7.639 | ||
| 35 | 6.2 | 7.894 | 75 | 5.7 | 7.257 | ||
| 36 | 6.5 | 8.276 | 76 | 5.8 | 7.385 | ||
| 37 | 6.2 | 7.894 | 77 | 5.7 | 7.257 | ||
| 38 | 6.1 | 7.767 | 78 | 5.9 | 7.512 | ||
| 39 | 6.4 | 8.149 | 79 | 5.5 | 7.003 | ||
| 40 | 6.5 | 8.276 | 80 | 5.9 | 7.512 |
Conclusion
PIXL has demonstrated a consistent picking pressure of under 9g/mm2, with an average pressure of approximately 7g/mm2. This has been attained through regulated contact pressure on every pick, ensuring protection of both the agar surface and microbial sample, whilst minimising the risk of contamination.
Ensure your colony picking is free from contamination caused by cell splashing
No pins, no problem. With Pinpoint picking technology PIXL detects the surface and regulates the contact pressure for every pick. This ensures that every single colony on your plate is picked, without damaging, missing or splashing cells all over the place.